March.30.2021
 Ingéniérie tissulaire, modèles de tissus en 3D pour l’industrie
Ingéniérie tissulaire, modèles de tissus en 3D pour l’industrie
EPISKIN supports ADEBIOTECH's Webinar on March 30th 2021 on Tissue Engineering for the Industry. Their application in Cosmetics, Pharmacology, Chemistry, Nutrition.
Please register to the webinar following this link: https://asso.adebiotech.org/webinaires/ingenierie-tissulaire-modeles-3d-pour-lindustrie-cosmetique/
February.2.2021
 FIRST EPISKIN ACADEMY WEBINAR, TUESDAY, FEBRUARY 02, 2021 02:00 PM CET
FIRST EPISKIN ACADEMY WEBINAR, TUESDAY, FEBRUARY 02, 2021 02:00 PM CET
ISO 10993-23 FOR BIOCOMPATIBILITY OF MEDICAL DEVICES
The new ISO 10993-23 for bio-compatibility of medical devices introduces the shift from in vivo to in vitro for the assessment of irritation potential of medical devices.
This is a change in mindset for which industry needs to prepare. The EPISKIN Academy webinar will cover irritation of medical devices, the scientific reasoning, the added value and the current limits of this new strategy. The training part of the Webinar – live broadcasting of the hands-on part of the assay - is a unique opportunity to discover all the tips and tricks of this protocol through an in laboratory session covering all the steps of the protocol.
Webinar’s agenda
Introduction (5 minutes)
Alain Alonso – Sales and Marketing Director &
Christian Pellevoisin - Scientific Director of EPISKIN Academy
EPISKIN – Presentation of the Company (5 minutes)
Alain Alonso – Sales and Marketing Director
Production and Quality Controls (5 minutes)
Anne-Sophie RIGAUDEAU – Production Director
ISO 10993-23, a shift from in vivo to in vitro assays for irritation of medical devices (25 minutes)
Christian Pellevoisin – Scientific Director of EPISKIN Academy
Live Demonstration of the in vitro assay for medical devices with 3D RECONSTRUCTED MODELS (10 minutes)
Christelle Videau - Scientific Customer Experience Manager
Q&A (30 minutes)
03:30 pm: End of the 1st EPISKIN Academy Digital Event
Hope to welcome you soon on our 1st websession online!
July.15.2020

Oral communication “Status update of new requirements ISO 10993 23” at MedTech Summit
The 2020 edition of the MedTech Summit will be on “EU MDR & IVDR: 2 YEARS TO GO. 2 REGULATIONS TO MEET. ARE YOU READY?”
Due to Covid-19 crisis this edition goes virtual. It will be held from October 12th to 16th, 2020.
Friday, 16 October 2020 15:00 - 15:25: Dr Christian Pellevoisin, Scientific Director of EPISKIN Academy, will present during the Biocompatibility for Medical Devices session:
Status update of new requirements ISO 10993- 23 ISO 10993-23 is a new standard expected this fall that will introduce the shift from in vivo to in vitro for the assessment of irritation potential of medical devices.
This is a change in mindset for which industry needs to prepare. The convenor of ISO/TC 194/WG 8 and chairman of AFNOR S92/J for the biocompatibility of medical devices will present the scientific reasoning, the added value and the current limits of this new strategy.
To discover how to subscribe to this event and full programme, please follow this link: MedTech Summit
April.30.2020
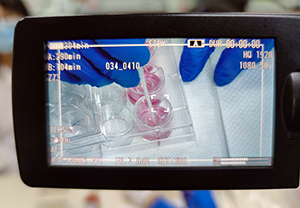
First live-broadcasting training event by Shanghai EPISKIN
Despite the impact from COVID-19 outbreak and restrictions on travel and conference gathering, the 9th EPISKIN Academy Workshop has just been successfully carried out through the live-broadcasting platform on Apr. 17th at Shanghai EPISKIN’s head office in Zhangjiang Hi-Tech park. This is also our FIRST online live-streaming show of technical training and product support to meet in vitro alternative testing related needs.
In our initiative to quickly adapt to the current circumstance with the agility to timely respond to our clients’ requests on technical training and support, given the fact that working activities cross sectors are recovering in China but regular on-site training still cannot be organized for the moment, this online workshop highly demonstrated the efficiency and effectiveness of such a new format for technical training and support.
Content of this 9th EPISKIN Academy Online Workshop:
1) Opening and Introduction
2) Review of in vitro Skin Irritation EpiSkin testing method (OECE TG439)
3) Demonstration of key technical steps of Skin Irritation Test using EpiSkin model and data analysis

4) Introduction of 3D skin models commercialized in China
5) Discussion Q&A

During the very special period we are all going through around the world, EPISKIN and the EPISKIN Academy are experimenting new way of communication using more and more digital technologies. This totally new way of sharing skin irritation protocols by live-broadcasting is a great success and we will soon provide other live sessions all over the world.
March.03.2020
 EPISKIN Academy at Nelson Labs seminar
EPISKIN Academy at Nelson Labs seminar
Dr. Christian Pellevoisin, Scientific Director at Episkin Academy, will present 'Assessing Alternatives to In-Vivo testing: Changing the Paradigm from In-Vivo to In-Vitro Toxicology' during the Nelson Labs seminar in Brabanthal, Leuven, Belgium, March 4 & 5, 2020.
Scientific program:
Leuven Open House 2020: The Year Of Change for the Medical Device Industry
Wednesday, March 04, 2020
12:00 Registration & welcome
13:10 Introduction – Eric Meyers (Senior VP EMEAA Nelson Labs) & Jos Bollen (Managing Director Nelson Labs Europe)
13:30 The EU Medical Device Regulation (MDR): 2020: The Year of Change – Henry Sibun (Henry Sibun Associates Ltd)
14:00 The new ISO 10993-18 Standard and its Impact on Chemical Characterization of Medical Devices – Dr Ted Heise (Vice President Regulatory and Clinical Services at MED Institute)
14:30 Linking ISO 10993-18 to ISO 10993-17 – Dr. Carsten Baun Senholt (ERT Chief Technical Officer at Saxocon)
15:00 Coffee break
15:15 MDR – View of a Raw Materials Supplier - Steve Duckworth (Global Head Marketing and Business Development at Clariant)
15:45 ISO 10993-18 in the MDR: understanding the restrictions and risk assessmentfor compounds which are carcinogenic, mutagenic, toxic to reproduction (CMR) or have endocrine-disrupting (ED) properties (section 10.4) – Dr. Annelies Vertommen (Senior E&L Expert Nelson Labs Europe)
16:15 Tour of the Laboratory Facility
17:00 Networking event
19:00 End of Seminar Day 1
Thursday, March 05, 2020
08:30 Registration
09:00 Introduction (Nelson Labs Europe)
09:05 MDR – Biocompatibility & reprocessing - Dr Gert Bos (Executive Director & Partner at Qserve)
09:45 The Biological Evaluation Plan (BEP): A crucial first step in the Biocompatibility evaluation of a Medical Device – Dr Sophie Michel (Associate Biocompatibility Expert)
10:15 Competent Authority perspectives on biocompatibility – Dr Pieter Van De Vijver (Non-Clinical Assessor, Medical Devices at FAMHPS)
10:45 Coffee Break
11:15 Assessing Alternatives to In-Vivo testing: Changing the Paradigm from In-Vivo to In-Vitro Toxicology – Dr. Christian Pellevoisin (Scientific Director at Episkin Academy, L’Oréal)
11:45 The need to Identify “Unknowns” from a Risk Management Perspective – Ron Brown (ex-FDA; now Director at Risk Science Consortium LLC)
12:15 Lunch
13:30 How to select a CRO for Chemical Characterization Testing – Dr Dennis Jenke (Principal Consultant Nelson Labs Europe)
14:00 Most Common Types of Observations in Regulatory Submissions of Chemical Characterization Results and how to address them – Dr. Piet Christiaens (Scientific Director at Nelson Labs Europe)
14:30 Break
15:00 Intro The Processing Cycle – Henry Sibun (Henry Sibun Associates Ltd)
15:15 Sterilization of your Medical Device – Annick Gillet (Technical Director EO Pharma – Sterigenics)
15:45 Reprocessing Validations of reusable Medical Devices (part 1) – Dr Lise Vanderkelen (Department Head Micro & Pharma Services Nelson Labs Europe)
16:15 Reprocessing Validations of reusable Medical Devices (Part 2) – Alpa Patel, Principal Scientist (Nelson Laboratories LLC)
16:45 Q&A (panel)
17:00 End of Seminar Day 2 – tour of the laboratory facility on-demand
*Times and topics subject to change
Direct link to Nelson Labs Seminar page:
www.nelsonlabs.com/
February.03.2020

Workshop on ocular irritation in Brazil
This Workshop will be the first complete training session on ocular irritation on SkinEthic HCE model for OECD TG492 and OECD TG437 in Brazil.
This program in partnership with InMetro follows the first training session in 2017 on skin irritation: https://www.youtube.com/watch?v=_daWZdYxh6Q
The workshop will be held in Inmetro, Campus de Metrologia e Inovação from February 03 to 07, 2020.
November.07.2019

ISO 10993: Are you biocompatible?
Tackle Testing Methods, Evaluations, Risk and Regulations With Competent Authority, Notified Body and Industry Guidance.
Dr Christian Pellevoisin from EPISKIN Academy will do a presentation on November 21st, CCIB, in Barcelona, Spain.
Assessing alternatives to animal testing: Changing the paradigm from in vivo to in vitro toxicology:
- Examining alternative available methods to predict biocompatibility
- Strategies for demonstrating the value of in vitro methods to predict biocompatibility
- Validation of skin irritation on 3D models to replace animal testing
- Latest developments in skin sensitization and perspectives for biomedical devices
- Assessing the need for positive materials with good human data to validate existing assays in vitro
Presentation will be held from 09:10 to 09:45.
If you want to learn more about presentation and book this session you can check website:
https://lifesciences.knect365.com/biocompatibility/speakers/christian-pellevoisin
October.16.2019
 Conference on Biocompatibility for Medical Devices US
Conference on Biocompatibility for Medical Devices US
The EPISKIN Academy, represented by Dr Christian Pellevoisin, will do a presentation during Biocompatibility for Medical Devices US.
October 24, 2019 in JW Marriott Chicago, Chicago, IL, USA.
Examining the latest developments in skin sensitisation and irritation testing:
- Validation of skin irritation on 3D models to replace animal testing
- Assessing how far away the industry is from having positive materials with good human data
- Assessing the need for good human data to validate existing assays in vitro
- Defining the right strategy for sensitisation and irritation testing
- Materials are on the market with no complaints/PMS requirements
Thursday, 24 October 2019 3:15pm - 3:50pm
To read more about the Congress: https://lifesciences.knect365.com
September.17.2019
 First International Forum on Quality Evaluation of Medical Devices and Biomaterials
First International Forum on Quality Evaluation of Medical Devices and Biomaterials
The Shandong Medical Device Product Quality Inspection Center held a lecture on the quality and safety evaluation of medical devices and biomaterials and the first international forum on quality evaluation of medical devices and biomaterials to further promote the improvement of the quality evaluation of biomaterials and medical devices, and promote the industry.
The first International Forum on Medical Device and Biomaterial Quality and Safety Evaluation jointly sponsored by Shandong Materials Biology Evaluation Branch and China Biomaterials Society will be held in Jinan, Shandong Province in September 2019.
This forum will bring together experts and scholars from different domestic and international review and approval, inspection and testing, research institutions and industrial enterprises to evaluate the quality of medical devices, focusing on the chemical characterization of medical devices and biological materials, biological evaluation and quality around medical devices.
Safety evaluation related to pre-clinical large animal experiments, integrating the three major cities of production, learning, research, the application of the latest hot topics, research progress and technical difficulties, such as academic reports, experience exchange and technology display.
In order to promote the development of medical device biomaterials quality and safety in the new situation, the new power of the committee will gather in Jinan to participate in the academic event!
September 23-25, 2019: Jinan Yinhuada Hotel ( No.1, Lvxi Caidang, Longxi Road, South Lixia District, E1, 0,531,058,999).
Download Chinese Meeting announcement here.
November.14.2018

EPISKIN Academy at the National Conference on Alternatives to Animal Experiments
EPISKIN Academy is invited to give a talk and a pre-conference training to the National Conference on Alternatives to Animal Experiments (NCAAE-2018) that will be held December 1st 2018 in New Delhi (India).
The Conference will be held at Jamia Hamdard, New Delhi, a Deemed to be University accredited in category ‘A’ by the National Assessment and Accreditation Council of India.
This Conference aims to discuss the perspectives on the current state of practices and existing strategies to promote the development of non-animal technologies in India (http://www.jamiahamdard.ac.in/NCAAE2018/NCAAE.htm)
On November 26th, Jamia Hamdard in collaboration with EPISKIN Academy under the aegis of Indian Society for Alternatives to Animal, organize a Hands-on Workshop to train 20 young scientists on Alternatives and in vitro skin Irritation according to OECD TG-439.
(http://www.jamiahamdard.ac.in/NCAAE2018/Hands-on%20workshop%20on%20reconstructed%20human%20epidermis_OECD%20TG%20439_Jamia%20Hamdard.pdf)
October.25.2018

EPISKIN at the Eurofins Human Safety Seminar
In Vitro Toxicology at a Glance: Today's Challenges, Solutions & Future Developments
EPISKIN is invited to give a lecture at the Eurofins Human Safety Seminar.
The Seminar will take place on November 29th, 2018 in the new laboratory and office building of Eurofins Munich, Robert-Koch-Straße 3a in Planegg near Munich, Germany.
Dr Pellevoisin, scientific director of EPISKIN Academy will give an overview of eye irritation methods with a focus on OECD TG492 for eye irritation of chemicals in the context of the Guidance Document on an Integrated Approach on Testing and Assessment (IATA) for Serious Eye Damage and Eye Irritation.
The second part of the talk will address the last advancement in skin sensitization testing strategies and added values of reconstructed human epidermis for this endpoint.
Follow this link to learn more about the Eurofins Seminar.
September.20.2018

ESTIV 2018 20th International Congress on In Vitro Toxicology
The 20th International Congress on In Vitro Toxicology will be presented from October 15th in Berlin, Germany.
Come and discover the new EPISKIN Academy presentation during the ESTIV congress:
Thursday, ocotber 18th
09.18 (OP 32):
Reconstructed human epithelia for in vitro biocompatibility of medical devices
Pellevoisin Christian (Lyon/FR)
To discover full programme please visit the ESTIV website.
May.23.2018.

|
The EPISKIN Academy at the IID 2018
|
During last IID (International Investigative Dermatology) congress which was held from May 16th to 18th, in Rosen Shingle Creek Resort, Orlando, Florida, the EPISKIN Academy presented two posters about the EPISKIN models:
01156 T-SKIN, a new industrial reconstructed human skin model for dermatological and cosmetics research and development
C. Pellevoisin, D. Lelièvre, M. Bataillon, N. Boyera, A. Rigaudeau, J. Ovigne, I. Besné, N. Seyler
Journal of Investigative Dermatology, Vol. 138, Issue 5, S196
Published in issue: May 2018
https://doi.org/10.1016/j.jid.2018.03.1170
01085 Cellular mechanistic investigation on antigen delivery by Viaskin® patch for epicutaneous immunotherapy with reconstructed human epidermis including Langerhans cells (SkinEthicTM RHE-LC)
V. Dioszeghy, C. Pellevoisin, M. Ligouis, F. Sahuc, V. Dhelft, L. Mondoulet
Journal of Investigative Dermatology, Vol. 138, Issue 5, S184
Published in issue: May 2018
https://doi.org/10.1016/j.jid.2018.03.1098
March.29.2018.
 |
EPISKIN ACADEMY poster reward at the 57th SOT
|
Brazil is the third country, after France and China, to produce locally an EPISKIN model. The SkinEthic RHETM model, used during the training, is produced through collaboration between L'Oréal and the Brazilian D'Or Institute for Research and Education (IDOR). EPISKIN, world leader of reconstructed human tissues for 25 years developed a wide range of expertise of applications of these models in different scientific, technological and regulatory contexts for both safety and efficacy purposes. In 2012, EPISKIN Academy have been created to share this knowledge and to prepare next generation of stakeholders to face the challenge of 21st century toxicology. EPISKIN Academy promotes and trains scientists all over the world to alternatives to animal testing in toxicology. To date more than 300 scientists have been trained in Europe, ASIA and America.
To view the video please follow this link: EPISKIN Academy Training Session in Brazil
June.21.2017

|
Inexo Symposium 2017 on Alternative Models
|
On July 7th, 2017 in Montpellier, France, the EPISKIN Academy will attend the Inexo Symposium 2017 on Alternative Models "in vitro, Ex ovo et Organismes": De la recherche aux applications dans les pathologies et le vieillissement.
Don't miss the EPISKIN ACADEMY presentation about Reconstructed Skin ("Peaux reconstruites") at Amphi B by Dr. Christian Pellevoisin.
To discover the complete scientific program please follow this link: https://inexo-2017.sciencesconf.org/
April.05.2017
|
8th World Congress on Toxicology and Pharmacology
|
On April 13-15, 2017 in Dubai, UAE, the EPISKIN Academy will attend the 8th Congress on Toxicology and Pharmacology.
Don't miss our keynote session on April, 15th, 2017 that will be held from 09:30 to 10:20: Reconstructed skin models and methods for hazard and risk assessment of chemicals and cosmetics presented byChristian Pellevoisin,
To discover the complete scientific program please follow this link: http://toxicology-pharmacology.conferenceseries.com/scientific-program
May.11.2016
 |
EPISKIN ACADEMY AT THE SID 2016 ANNUAL MEETING
|
The Episkin Academy will attend the SID 2016 Annual Meeting that will be held at the Westin Kierland Resort & Spa in Scottsdale, Arizona, from May 11 to 14, 2016.
'The use of SkinEthic reconstructed human epidermis (RHE) to study immunocompetent cells trafficking and recruitment between skin compartments' will be presented during poster session on Thursday, May 12 and Saturday, May 14.
To learn more about SID annual meeting and scientific program, please follow this link: http://www.sidnet.org/2016annualmeeting
January.08.2016
 |
EPISKIN Academy created in China
|
L'Oreal China and Shanghai EPISKIN Biotechnology Ltd. announced the creation of the EPISKIN Academy in China.
Through training and technical collaborations, the Academy will work with Chinese scientists, key opinion leaders, students and future stakeholders in terms of scientific and regulatory issues for alternatives to animal testing in field of safety, toxicology and efficacy as well as in other relevant areas.
To further promote the reconstructed skin models through marketing, L'Oréal China and EPISKIN SA created Shanghai EPISKIN Biotechnology Ltd. in 2014 to produce reconstructed skin models according to high quality standards in the industry. The models will be used primarily for research on skin tissue engineering and testing. This time the creation of the EPISKIN Academy will certainly play an important role in promoting in vitro skin models in the industry.
"In China, the Academy will work closely with various parties to promote the reconstructed skin models and alternative methods in China, and will strive to become a key scientific resource to further stimulate innovation in the Chinese cosmetics industry" said Dr. Christian Pellevoisin, scientific Director of the EPISKIN Academy.
August.25.2015
 |
The Episkin Academy at CIFARP
|
Dr Christian Pellevoisin, Scientific Director of the Episkin Academy, has been invited by the Organizing Committee of CIFARP to be a speaker at 10th Congress of Pharmaceutical Sciences that will be held in Ribeirão Preto – São Paulo -Brazil, from September 06-09, 2015.
This year the theme is “Translating science into health pipeline” :
September 8th - 10:30-12:30 - S9.2: Episkin and the application of reconstructed human 3D models in toxicology and pharmacology.
CIFARP 2015 will be a unique opportunity to discuss the latest trends and scientific breakthroughs in the pharmaceutical sciences field. About 1400 national and international participants are expected, including researchers, professionals, as well as postgraduate and undergraduate students.
For more information consult the website of the congress: http://www.cifarp.com.br/scientific-program.php
March.06.2015
 |
Episkin Academy training: ENMV Sidi Thabet- Tunisia, february 23-26, 2015
|
The National School of Veterinary Medicine Sidi Thabet organized a training in animal experiments which theme was: "From in vivo models to cell models".
This training took place in ENMV of Sidi Thabet (University of Manouba) and lasted 4 days from 23 to 26 February 2015.
34 participants (biology doctoral students, teacher-researchers, veterinarians, industrial and control pharmacists) were informed of the new cell technologies and alternative methods which aim to replace animals in drug-Toxicological, chemical and cosmetics testing. Practical workshops and round table helped to promote exchanges and networking with European partners.
Main objectives:
- Promote good practice and ethics in animal experimentation based on the principle of the 3Rs (Replacement, Reduction, Refinement).
- Presentation and training on new cellular technologies (2D and 3D) on two organs: liver and skin (primary liver cells, liver microsome, skin cells ...) in the pharmaco-toxicological and pharmaceutical research.
- Present the situation in Europe and internationally of the new technologies (in silico, in vitro, omics "adverse outcome pathways") in the academic and regulatory (REACH) context.
This training also offers an opportunity to discuss collaborative projects (medium and long term) with the scientific and medical community as well the pharmaceutical industry in the new research program called European "Horizon 2020" and beyond.
The Episkin Academy presented the Pharmacotoxicology applications of the 3D reconstructed human epidermis models during the round table. This presentation was followed by a training session on the use of our epidermal tissues.
Scientific Program:
|
9h00 – 12h00 |
14h00 – 17h00 |
Monday
Feb 23, 2015 |
- Welcome and registration
- Ethics and Welfare AL
- Guiding principles CIOMS / ICLAS
- Recommendations of the OIE (OIE)
(Ouajdi Souilem, ENMV Sidi Thabet) |
Workshop 1
Demonstrations on in vivomodels
Team Azal / ENMV / IPT |
Tuesday
Feb 24, 2015 |
- Place of alternative methods in EA
(Sarrah M'barek, ISSBAT, Tunis)
-Internationally trendof the 3Rs and funding opportunities
(FrançoisBusquet coordinator CAAT, EU, Belgium)
- Zebrafish Model and Applications
(François Busquet, EU Brussels) |
Workshop 2
REACH and MA Dissemination in Tunisia
Intervention of the Chemistry technical center
Setting up a test platform (François Busquet) |
Wednesday
Feb 25, 2015 |
- Cell culture: advantages and limits
(Olfa Masmoudi, FST, Tunis)
- In vitro toxicology - pharmacology
(Christophe Chesné, France)
- Applications in pharmacotoxicology of 3D reconstructed human epidermal models
(Christian Pellevoisin, France) |
Workshop 3
2D models: liver cells
HepaRG BIOPREDIC (Christophe Chesné) |
Thursday
Feb 26, 2015 |
Workshop 4
3D models: skin cell culture
Episkin Academy - Lyon (Christian Pellevoisin) |
Synthesis, debate and Discussion
Distribution of certificates
Closing Ceremony |
Workshop & training session in India
|
Workshop & training session in India
|